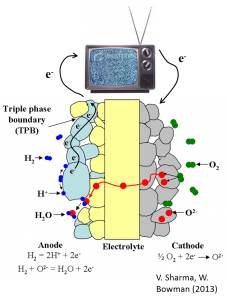
 Fuel cells are a promising technology for efficient utilization of fossil fuels, hydrogen and biofuels. In a solid oxide fuel cell (SOFC), a cathode reduces gaseous oxygen to oxygen ions which diffuse through an electrolyte to an anode where they oxidize a fuel resulting in the release of electrons which can drive an external circuit. SOFCs are attractive because they can directly utilize a variety of different fuels (hydrogen, hydrocarbon, etc…), and high temperature operation enhances the electrocatalysts’ resistance to poisons. The anode, cathode and electrolyte are composed of ceramic components, and high operating temperatures can lead to material stability issues which negatively affect cell performance. Many of these problems may be mitigated by developing intermediate temperature solid oxide fuel cells (IT-SOFC) that operate in the temperature range 400-600oC. Doped cerium oxides are potential candidate materials for electrolytes and anodes because of their desirable electrical and catalytic properties at intermediate temperatures.
Fuel cells are a promising technology for efficient utilization of fossil fuels, hydrogen and biofuels. In a solid oxide fuel cell (SOFC), a cathode reduces gaseous oxygen to oxygen ions which diffuse through an electrolyte to an anode where they oxidize a fuel resulting in the release of electrons which can drive an external circuit. SOFCs are attractive because they can directly utilize a variety of different fuels (hydrogen, hydrocarbon, etc…), and high temperature operation enhances the electrocatalysts’ resistance to poisons. The anode, cathode and electrolyte are composed of ceramic components, and high operating temperatures can lead to material stability issues which negatively affect cell performance. Many of these problems may be mitigated by developing intermediate temperature solid oxide fuel cells (IT-SOFC) that operate in the temperature range 400-600oC. Doped cerium oxides are potential candidate materials for electrolytes and anodes because of their desirable electrical and catalytic properties at intermediate temperatures.
While considerable progress has been made towards the goal of accomplishing IT-SOFCs, there are a number of important scientific and technical issues regarding the behavior of doped cerias for intermediate temperature applications. The ionic conductivity of these oxide electrolytes is strongly influenced by composition, grain boundary structure and defects. We are synthesizing electrodes with different compositions and structure using simple spray drying techniques. Grain boundaries are particularly problematic because they may hinder the flow of oxygen ions through polycrystalline materials. We are exploring the effect of grain boundary doping as a method to improve ionic conductivity. We determine grain boundary structure and composition using aberration corrected TEM/STEM imaging and monochromated electron energy-loss spectroscopy in the electron microscope. We correlate the atomic level structure of the materials with ionic conductivity determined with impedance spectroscopy.
For fuel cell anodes, it is desirable to convert hydrocarbon fuels into CO and H2 which are then oxidized electrocatalytically to generate electricity. This fuel conversion process is know as internal reforming. Consequently electrodes have being engineered to have distinct functional layers for reforming, current collection or electrocatalysis. The advantage of internal reforming is that it could considerably reduce the balance of system cost but carbon deposition onto the anode from carbonaceous fuels may degrade performance or even destroy the electrode.
It is important to find materials that can reform a variety of different fuels directly on the anode without forming significant carbon depositions. Doped ceria has desirable reforming properties and can significantly reduce the amount of carbon deposited onto anodes during operation in hydrocarbon fuels. However an atomic level understanding of the mechanism by which doped cerias can protect the metal component of the cermet has not yet been determined. In situ environmental transmission electron microscopy (ETEM) is being performed to directly observe carbon deposition onto cermets in hydrocarbon atmospheres. The relationship between the cermet structure, composition, morphology and the local carbon deposition is being investigated to point the way forward to developing improved cermets for use with carbonaceous fuels.
Current Projects