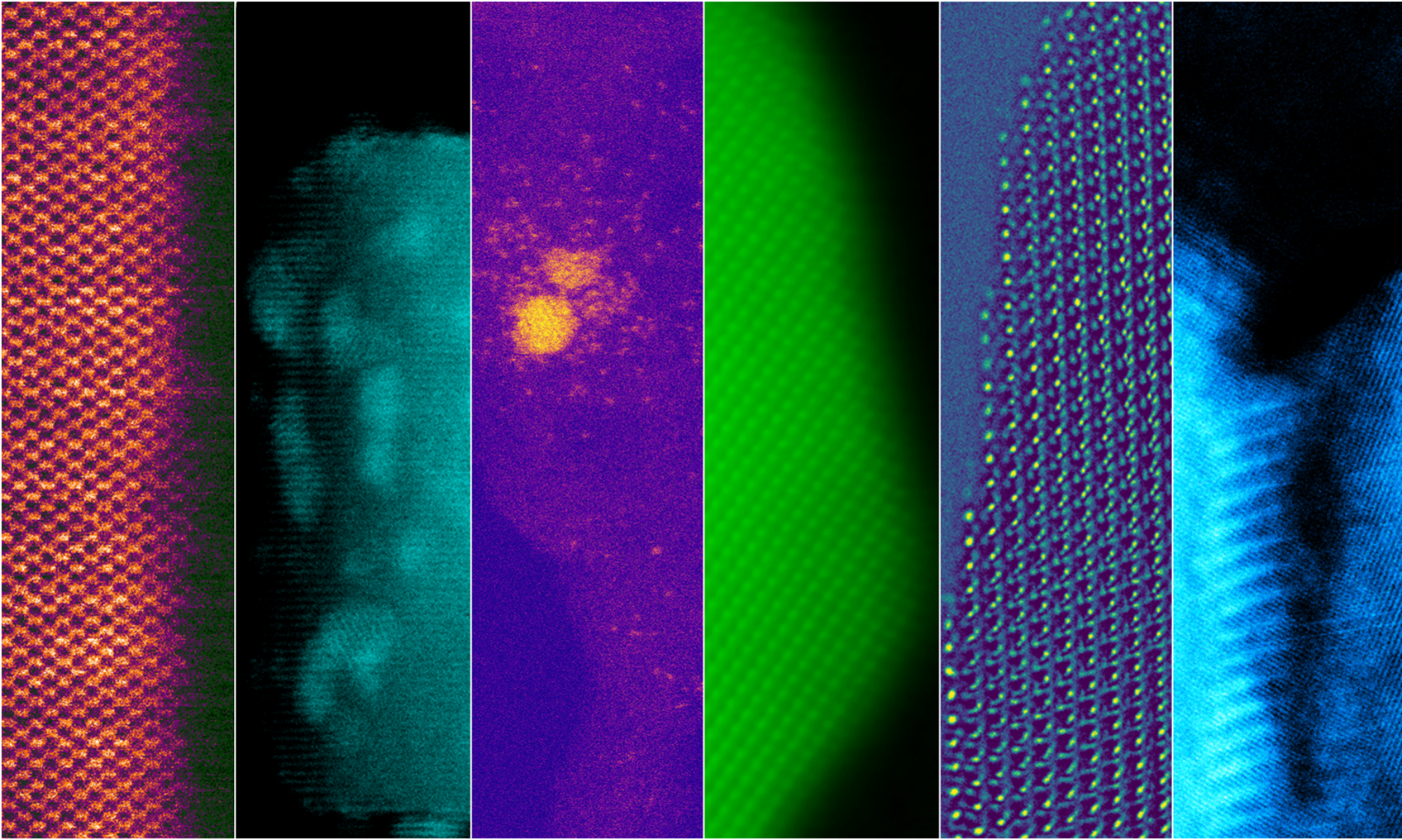
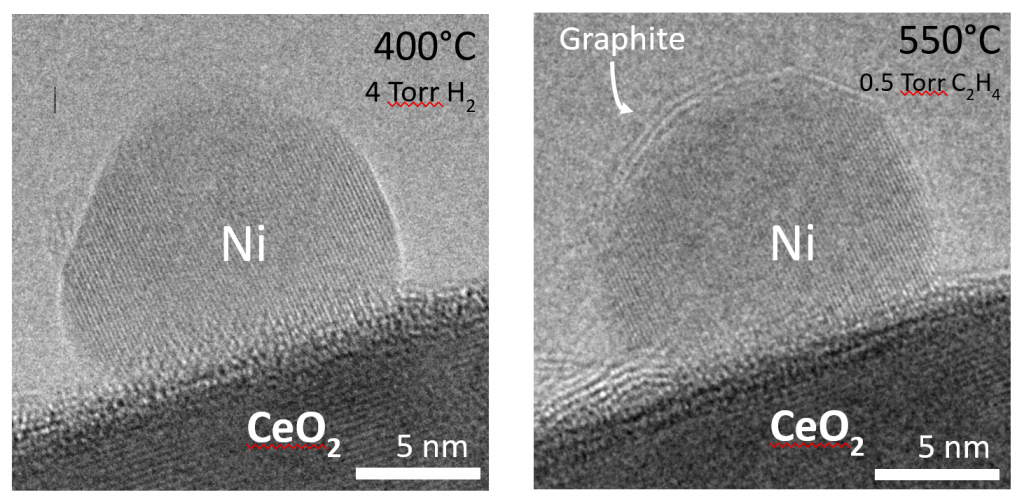
Our research focuses on Ni/Gd co-doped ceria (CeO2) anode reforming catalysts. Since Ni catalyzes carbon growth, when carbonaceous fuels such as natural gas are employed under certain conditions, carbon can deposit onto Ni surfaces. This can significantly degrade or even destroy the anode by carbon diffusing into the bulk Ni metal and causing fractures in the structure. Ni particles can also be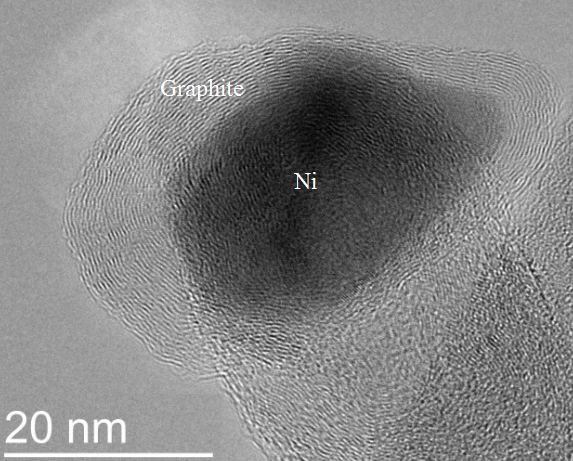 ome encapsulated ith graphite layers which can render the atalyst inactive. An image of graphite encapsulating a Ni nanoparticle is shown to the right. Furthermore, ceria is known to be an oxidation catalyst and thus the combination of ceria and Ni may result in suppressed carbon formation. We are interested in fundamental investigations of the carbon deposition mechanism on Ni/doped ceria catalysts.
ome encapsulated ith graphite layers which can render the atalyst inactive. An image of graphite encapsulating a Ni nanoparticle is shown to the right. Furthermore, ceria is known to be an oxidation catalyst and thus the combination of ceria and Ni may result in suppressed carbon formation. We are interested in fundamental investigations of the carbon deposition mechanism on Ni/doped ceria catalysts.
Ex situ and in situ environmental transmission electron microscopy (ETEM) has been employed to investigate the atomic level processes that result in carbon formation. Shown below are two in situ images of graphite layers being deposited onto a Ni particle which is on a ceria support.
Current experiments include observing carbon deposition due to different hydrocarbon gases and using spectroscopy to monitor changes in the oxidation state of the ceria support. This will help us to understand why ceria can inhibit carbon deposition on Ni particles in some hydrocarbon gases but not others.
Gaining a fundamental understanding of the relationship between the material’s structure, catalytic properties and carbon deposition will help enable optimization of SOFC anode material.
Personnel
 Ethan Lawrence is a fourth year graduate student in the Materials Science & Engineering Ph.D. program. He gained his bachelor’s degree from Coe College in Cedar Rapids, IA majoring in physics and math.
Ethan Lawrence is a fourth year graduate student in the Materials Science & Engineering Ph.D. program. He gained his bachelor’s degree from Coe College in Cedar Rapids, IA majoring in physics and math.