(Funded by the National Science Foundation and the Department of Energy)
Cerium-based oxides are important components of many catalysts they can supply or remove oxygen during redox reactions. During catalysis, oxygen vacancies are constantly created and then refilled in the oxide nanoparticles and the activity of the catalyst is directly related to the ease with which these vacancies can be generated. The high vacancy concentration may lead to substantial phase transformations in the active form of the material. We are engaged in studying the activity of cerium-based oxides with potential applications to solid oxide fuel cell anodes. Using environmental transmission electron microscopy we are able to determine the activity on individual nanoparticles and determine the structure and composition of active phases. In parallel with our experimental work, we are using density functional theory to determine the effect of different dopants on the oxygen vacancy formation energy at both the bulk, surface and interface regions.
Recent Publications from This Project
R. Wang, P. A. Crozier*, R. Sharma, and J. B. Adams (2006), “Nanoscale Heterogeneity in Ceria Zirconia with Low Temperature Redox Activity “, J.Chem.Phys. B, B 110 (37): 18278-18285
R. Wang, P. A. Crozier*, R. Sharma, and J. B. Adams (2008), “Measuring the Redox Activity of Individual Catalytic Nanoparticles in Cerium-Based Oxides” Nanoletters, 8(3), 962.
P. A. Crozier*, R. Wang, and R. Sharma, (2008), “In situ environmental TEM studies of dynamic changes in cerium-based oxides nanoparticles during redox processes” Ultramicroscopy 108 1432-1440
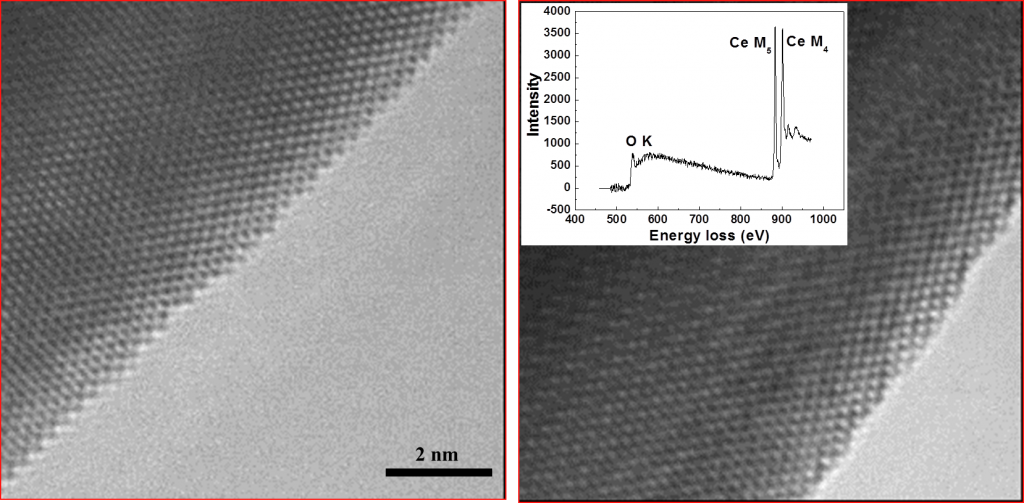
Changes in the structure of a cerium oxide nanocrystal during reaction in a hydrogen atmosphere. Left image is electron micrograph recorded at 600oC in H2. Right image is same area of crystal recorded at 725oC in H2. At 700oC some of the oxygen in the crystal reacts with gaseous hydrogen to make water leaving behind a large number of oxygen vacancies in the crystal. These oxygen vacancies order giving rise to a superstructure leading to the coarse fringe pattern shown in the right-hand figure. We can use electron energy loss spectroscopy (insert) to probe the chemical change taking place in nanoparticles during catalysis.