Solid oxide fuel cells (SOFCs) are one type of fuel cell whose performance is strongly dependent on the ability of oxygen ions to migrate through an ion-permeable membrane called an electrolyte. Current commercially available SOFCs utilize yttria-stabilized zirconia (YSZ) electrolytes which require high operating temperatures (~1000°C) to achieve high ionic conductivity. This temperature requirement severely limits potential component material selection. A potential alternative to YSZ electrolytes are doped CeO2 (ceria) electrolytes which exhibit considerable ionic conductivity at intermediate temperatures (500°C − 700°C.)
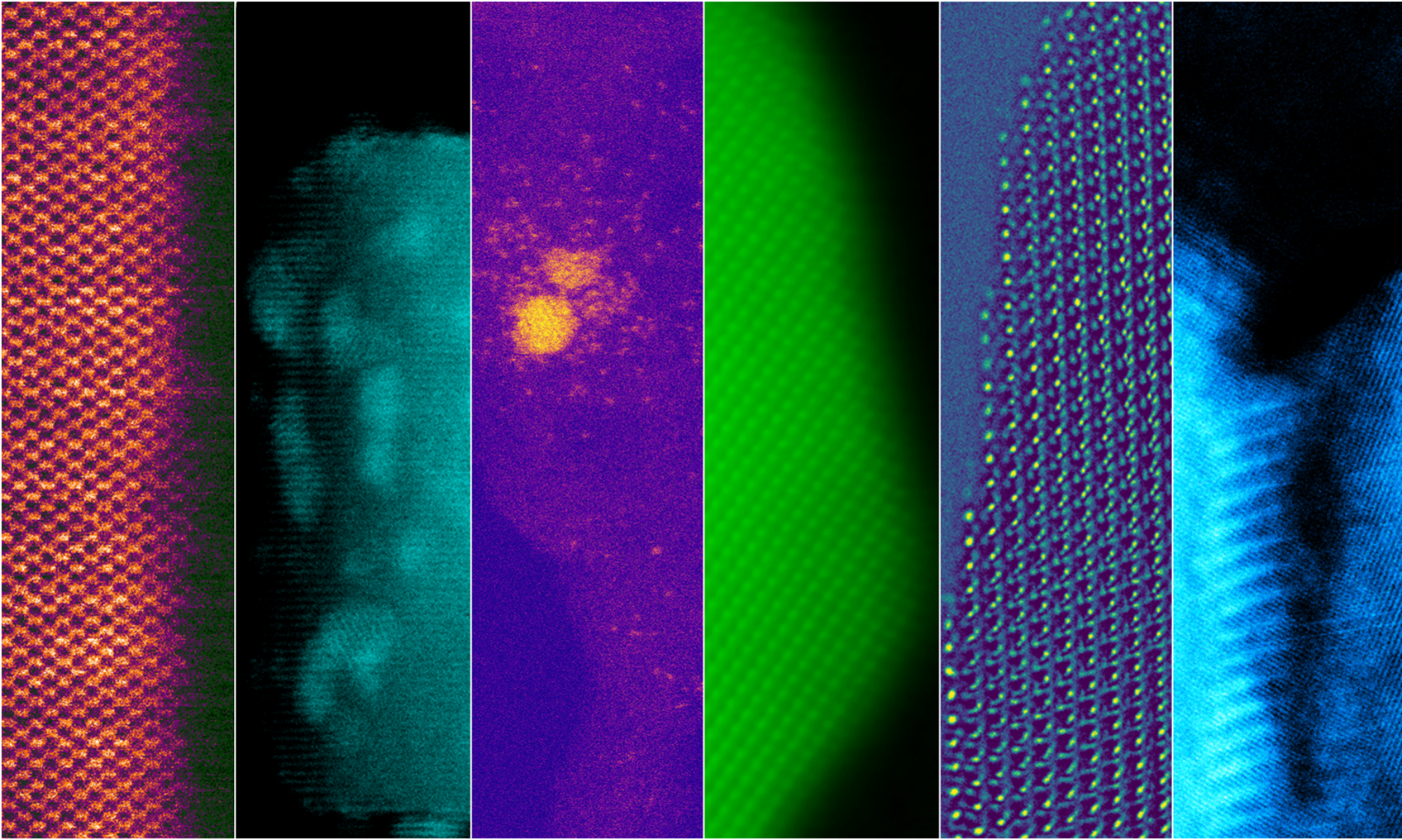
In an effort to understand fundamental materials phenomena which govern the behavior of SOFCs, we utilize high resolution imaging and nanospectroscopy in the electron microcsope coupled with simulations to correlate micro- and nano-scale structure and chemistry with electrical properties of ceria-based solid electrolytes.
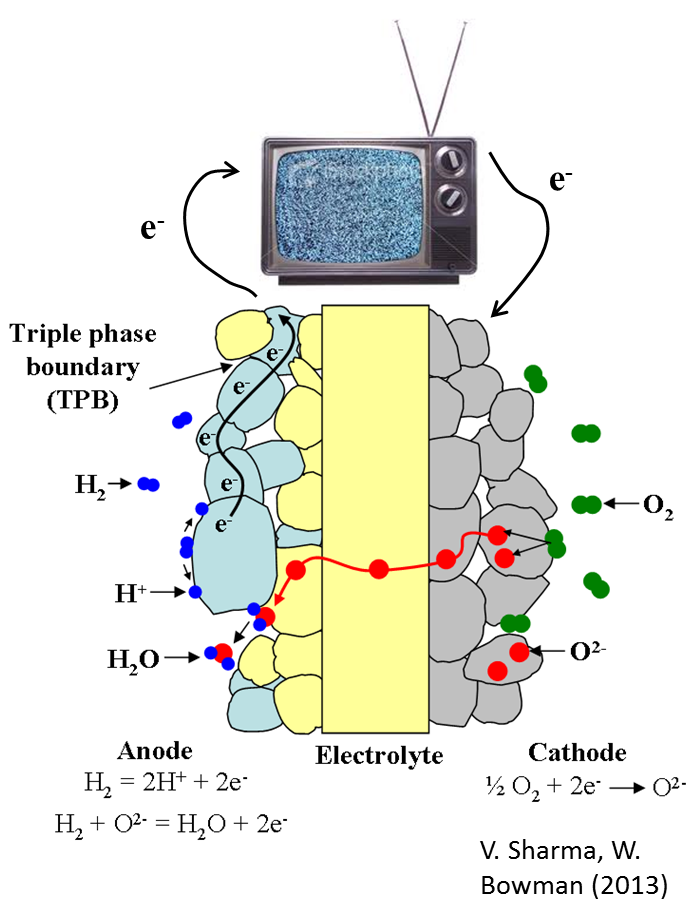
The diagram to the right illustrates the SOFC operating principles. Oxygen molecules (O2) are reduced by electrons (e-) at the cathode to form oxygen ions (O2-), which then diffuse through the electrolyte to the anode where they participate in the oxidation of fuel molecules at triple phase boundaries. Hydrogen gas (H2) is shown as the fuel in this diagram; however, an attractive feature of the SOFC is its ability to utilize a diverse fuel stream including hydrocarbon fuels such as methane, the chief component in natural gas.
Material Synthesis
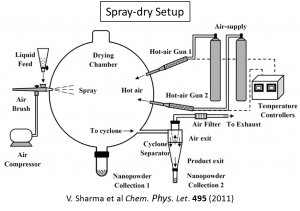
All material synthesis is performed in house using an inexpensive and relatively simple synthesis technique called spray drying. By this method an aqueous solution is sprayed as a fine mist into a reaction vessel where it is introduced to a stream of heated air. The water rapidly evaporates producing powders which are compositionally mixed at the nanometer scale. Below is a diagram of the spray drying apparatus used in our lab.
For more information about spray drying refer to Sharma, V., Eberhardt, K. M., Sharma, R., Adams, J. B. & Crozier, P. A. A spray drying system for synthesis of rare-earth doped cerium oxide nanoparticles. Chemical Physics Letters 495, 280–286 (2010).
Characterization of Electrical Properties
We prepare all electrolytes from powders synthesized in house. We characterize the electrical properties of these electrolytes using AC-impedance spectroscopy performed in air at temperatures up to 1000°C. This technique allows us to determine the unique contributions of grains and grain boundaries to the overall conductivity of the electrolyte – an important capability when considering the effects of highly localized structural and compositional features.
Electron Microscopy
For atomic level characterization of the structure, composition, and bonding in the electrolytes, we employ scanning (SEM) and transmission electron microscopy (TEM) techniques such as high resolution imaging, energy dispersive x-ray spectroscopy (EDX), and electron-energy loss spectroscopy (EELS).
Personnel
 William Bowman graduated with a Materials Science and Engineering Ph.D. in 2016 . He received his bachelors in the same field from Arizona State University in 2012.
William Bowman graduated with a Materials Science and Engineering Ph.D. in 2016 . He received his bachelors in the same field from Arizona State University in 2012.