In situ TEM
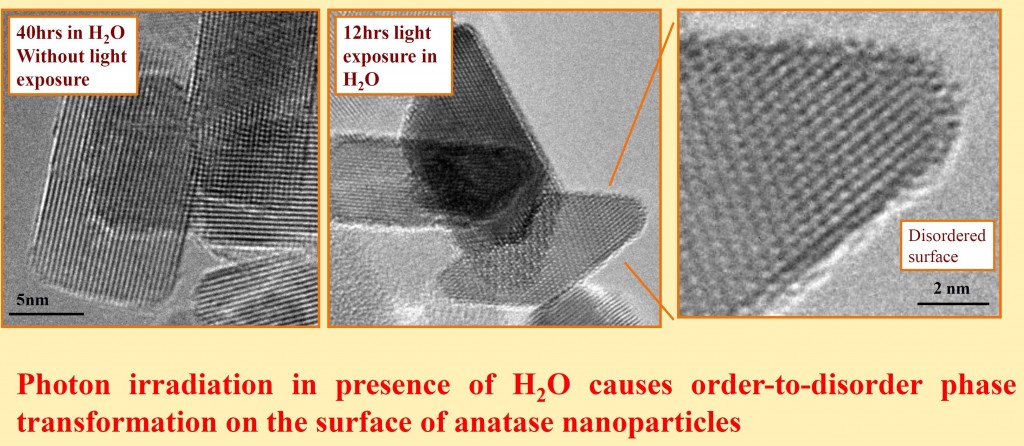
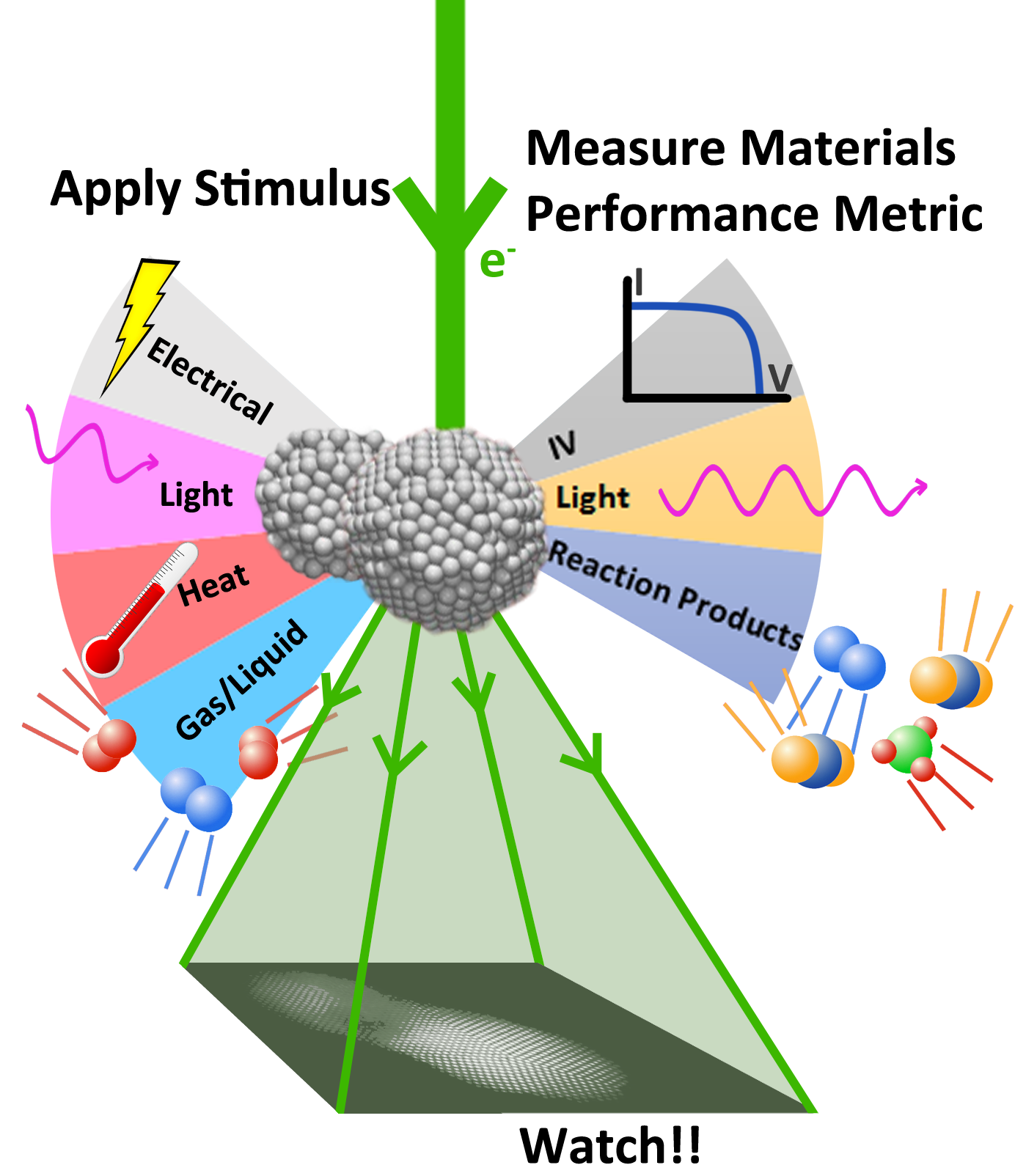
In technological applications, a material will be exposed to some external stimuli during operation. Heterogeneous catalysts, for example, are immersed in reactive gasses at high temperatures; batteries experience an applied load. The stimulus may take many forms, including elevated temperature, reactive gas or liquid environment, mechanical stress, light exposure, or magnetic and/or electric field application. Understanding and developing material systems for technological applications requires investigating the behavior of the material in the situation in which it is used. The type of TEM developed to study dynamic changes taking place in materials subject to external stimuli is called in situ TEM.
An advanced form of in situ TEM allows us to understand the complex behavior of energy-related materials exposed to a combination of heat, gas and/or light. This type of in situ TEM is called environmental TEM, and when employed in aberration-corrected, atomic-resolution microscopes, it allows us to follow the atomic phenomena taking place in reactive gas environments. Using this approach, we can record movies of atoms moving over the surface of nanomaterials and observe atomic dynamics in real time.
Operando TEM
Atomic-resolution in situ TEM can offer incredible insight into material phenomena at the finest scale. However, to obtain a complete picture of a material’s behavior under technologically relevant environmental stimulation, we must also know how well the material performs its technologically relevant function. That is to ask: how active is the catalyst? how powerful the battery? Operando TEM is a new technique being developed to better understand how the structure of a material is related to its functional performance.
In the field of catalysis, many good catalysts are not fully understood. While they are known to promote certain reactions, the fundamental reasons for their high catalytic activity remain unknown. If the mechanisms for these reactions can be fully elucidated, it becomes possible to intentionally design other catalyst materials with intelligent structures that take advantage of this knowledge. These new materials might be more highly active/selective, cheaper, or more sustainable than current catalysts.
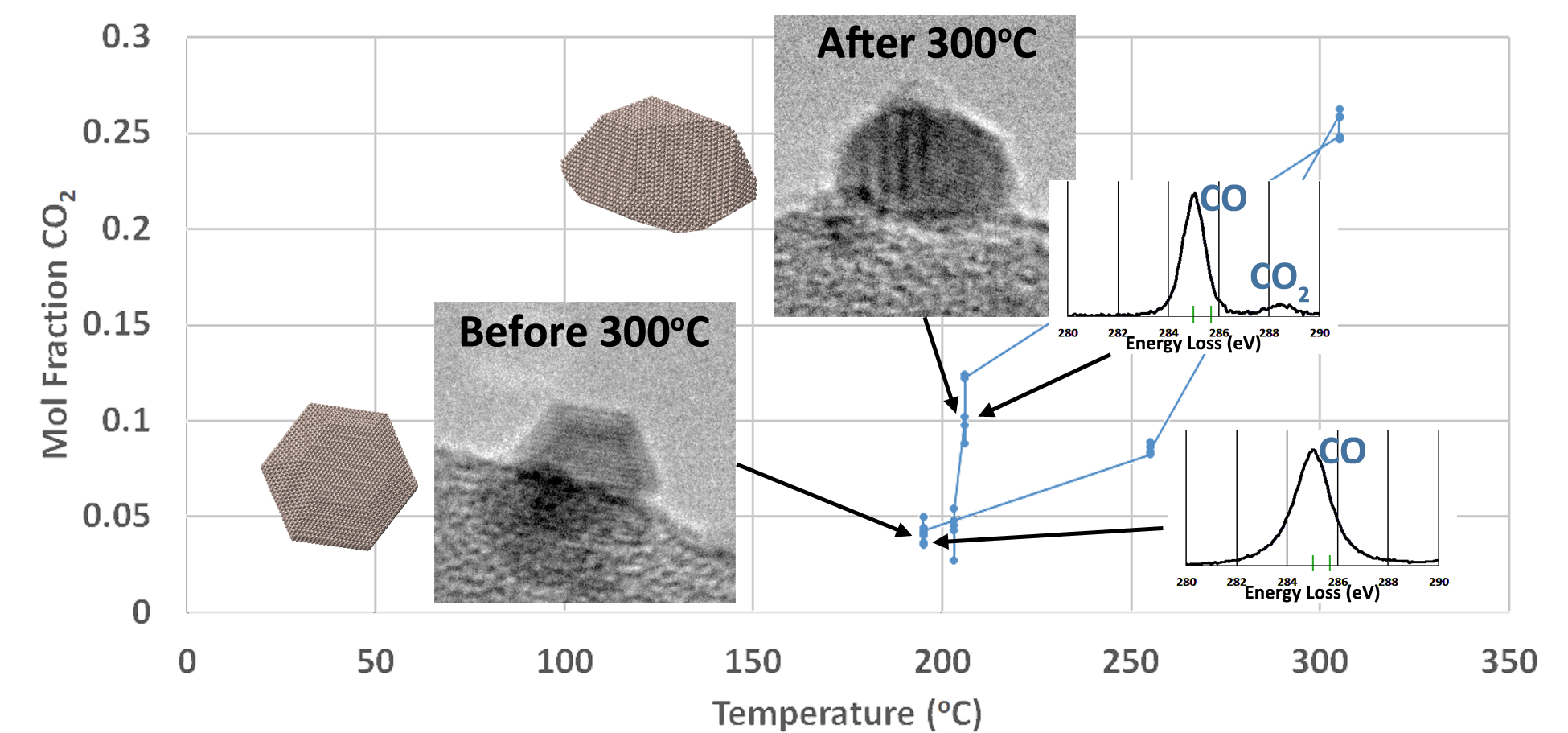
In an operando experiment we observe the catalyst structure using an environmental TEM providing reactive conditions for the catalyst. The catalytic reaction occurring inside the microscope is monitored by analyzing the gas composition in the microscope using both mass spectrometry (RGA) and electron energy-loss spectroscopy (EELS). The gas composition determined by RGA and EELS can be unambiguously correlated with images of the catalyst at that particular condition.
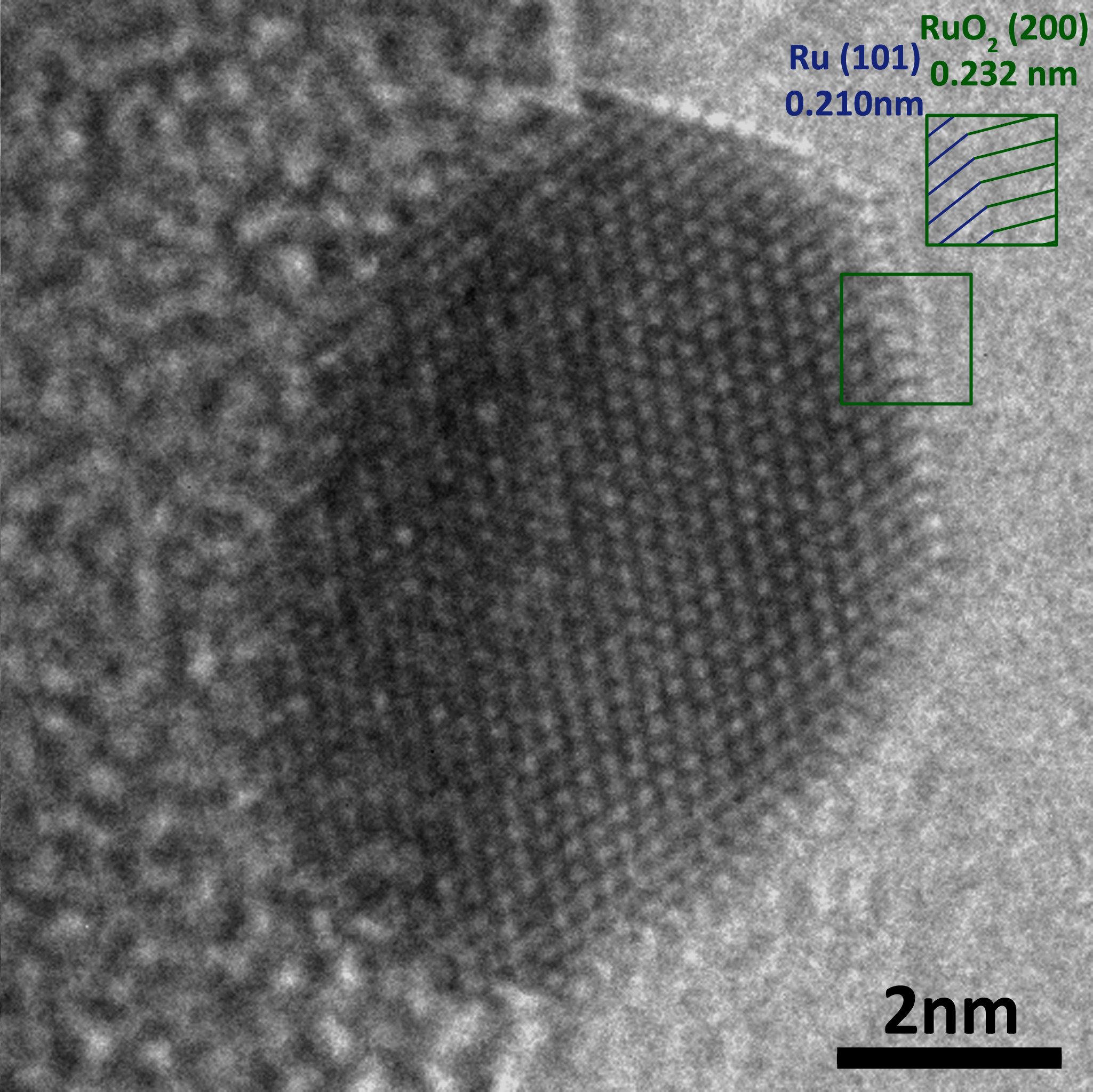
Eventually, such correlation should help us to determine structural features which are responsible for high catalytic activity, thus informing catalyst design.