[under construction]
Solar fuel technologies use catalysts to convert low-energy molecules into fuels via artificial photosynthesis. An essential component of solid oxide fuel cells is an oxygen ion conducting material; ion conductivity is related to oxygen vacancy concentration. TiO2, or titania, is an important model material for studying both catalytic and ion conducting functionalities. A fundamental knowledge of the underlying structure and functionality of TiO2 is obtained using the complementary techniques of transmission electron microscopy (TEM) and electron energy loss spectroscopy (EELS). Transmission electron microscopy gives information about the positions of the atoms and electron energy loss spectroscopy gives information about the electronic structure and bonding of the material. The FEFF software developed by researchers at the University of Washington calculates theoretical EELS. FEFF is being used to develop a method for mapping oxygen vacancy concentration in TiO2 and to study the changes in surface structure of TiO2 during chemical reactions. She hopes that the techniques developed can be applied to study a wider range of materials for sustainable energy technologies.
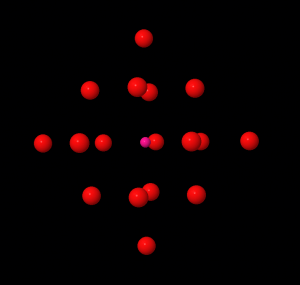
The figure above shows the oxygen sublattice of the anatase form of titania on the left. An oxygen vacancy in any one of the red sites will affect the energy loss spectrum from the pink central oxygen atom. The graph on the right shows a simulated energy loss spectrum for anatase with a 5% oxygen vacancy concentration (blue line) compared to anatase with no oxygen vacancies (red line). The theoretical calculations predict a drop in the intensity of the first peak as well as a slight shift to higher energy and splitting into two peaks. It remains to be seen whether these predictions are observed experimentally.